In-Country Clinical Caretaker (ICCC) Services
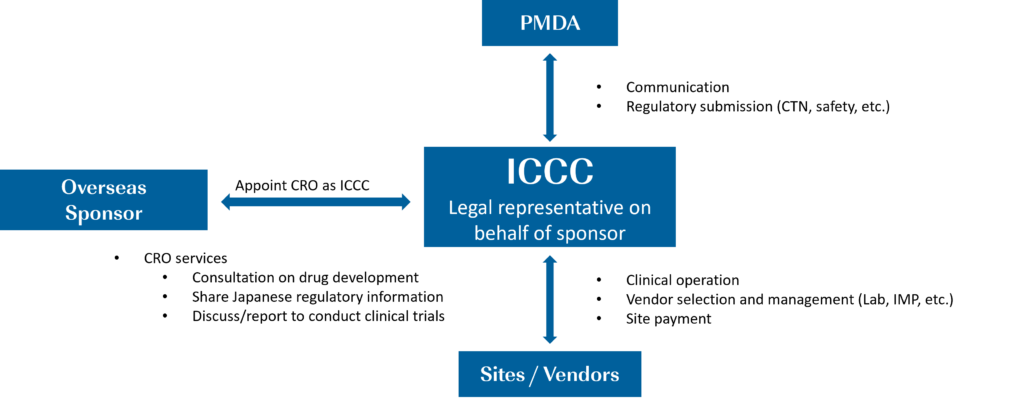
What are In-County Clinical Caretaker (ICCC) Services?
At CMIC Group, we can act as an In-Country Clinical Caretaker (ICCC) for overseas sponsors. Individuals who intend to sponsor a clinical trial in Japan, while residing outside of the country shall appoint an ICCC residing in Japan, who is eligible to sponsor the clinical trial on their behalf.
JCROA (Japan CRO Association) defines ICCC as below:
“In order to take the necessary measures to prevent the occurrence or spread of health hazards due to drugs used in the clinical trial, a person who intends to sponsor a clinical trial and resides outside Japan shall appoint a person eligible for sponsoring the clinical trial on behalf of the person who intends to sponsor a clinical trial from among persons residing in Japan (including the head of a Japanese business office of a foreign company) to have him or her (hereinafter referred to as “Clinical Trial In-Country Representative*”) conduct the procedures for sponsoring the clinical trial (J-GCP Article 15). *: Clinical Trial In-Country Representative is referred to as an In-country Clinical Caretaker (ICCC) in this document.”
Reference: https://www.jcroa.or.jp/english/essential-considerations-for-iccc/
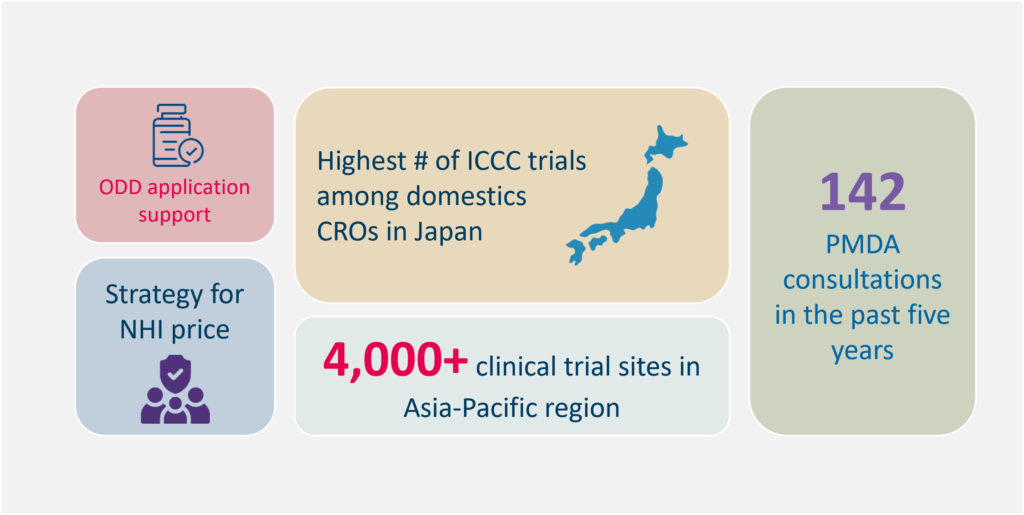
Our Edge in In-Country Clinical Caretaker Services
Track record of successful contracted projects from overseas Sponsors