Contract Development and Manufacturing Services
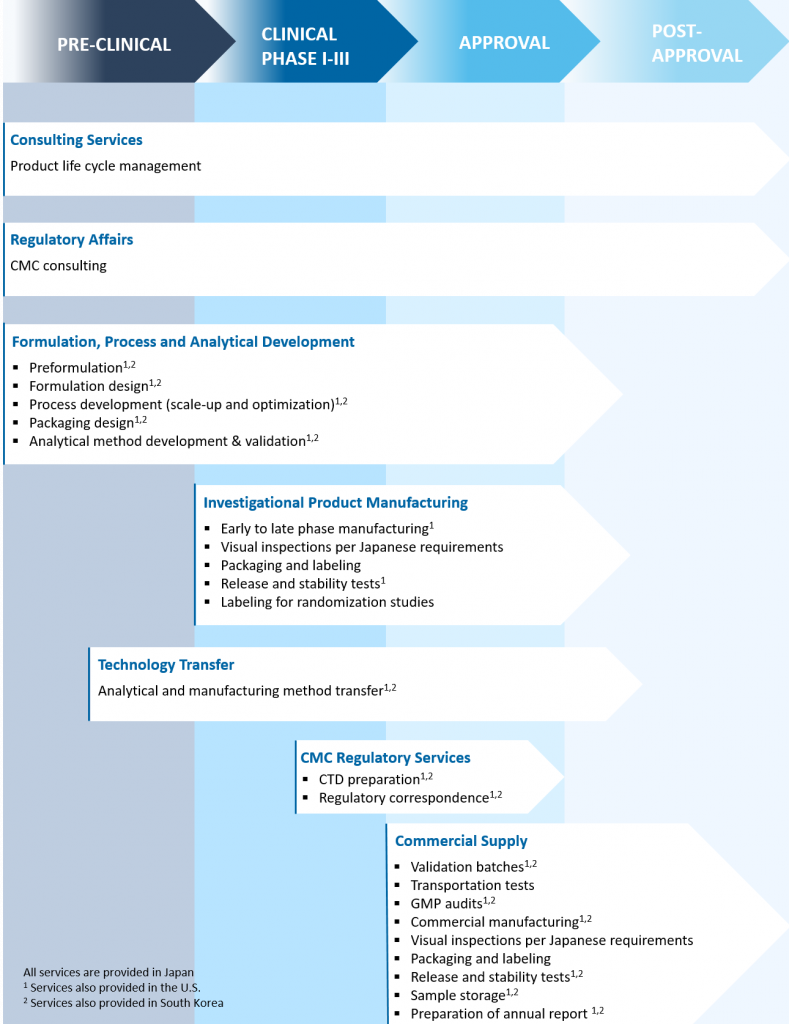
Global End-to-End CDMO Solutions
Bridging the US, Japan and Asia with Contract Development & Manufacturing Solutions
As the largest and first CRO in Japan, CMIC Group also has drug development expertise with advanced manufacturing and packaging platforms to help accelerate your drug development timeline. Whether you are launching new drugs in the U.S. or considering new market entry for the Japanese market, we are your partner for global product development with the ability to seamlessly transfer technologies across borders for manufacturing. With our broad offerings, we can help you tie your development and commercialization needs in the West with the East.
Our Edge in Contract Development & Manufacturing