Clinical Monitoring, From Feasibility Assessment to Site Closeout
CMIC is the first and largest CRO in Japan with 1,200+ Clinical Research Associates (CRAs) including Asia-Pacific. Our clinical operation services include: feasibility, site selection, contract negotiation and execution with clinical sites, clinical monitoring (including risk-based monitoring and remote monitoring), supplying and retrieving investigational products, collection and checking case report forms, and processes for clinical studies closeouts. We work closely with investigators and site staffs to execute the clinical trials smoothly following GCP and other regulations. We have multinational clinical study trial management expertise, and our clinical operations services are available in Japan, South Korea, Singapore, Taiwan, Malaysia, Hong Kong, the Philippines, China, Vietnam, Thailand, Indonesia, Australia and New Zealand.
Our Edge in Clinical Operation
Vast experience and expertise in Japan and broader Asia-Pacific
- We have the largest team of CRAs in Japan and also have highly experienced CRAs in Asia-Pacific region. With our extensive experience and expertise, we support our clients’ clinical trials in various therapeutic areas.
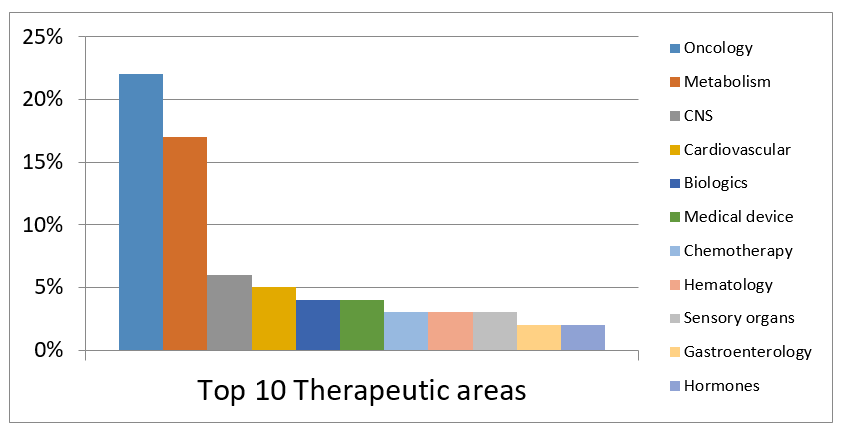
Services
- Site selection
- Clinical monitoring (including risk-based monitoring and remote monitoring)
- Contract negotiation and execution with clinical sites
- Trial fee payment to clinical sites
- Supplying and retrieving investigational products
- Collection and checking case report forms
- Processes for clinical studies closeouts