[Blog] How Japan is Advancing Rare Disease Projects: Opportunities for Global Biopharma
Rare diseases, often called “orphan diseases,” affect small patient populations but represent some of the greatest unmet medical needs worldwide. For companies in biopharma and biotech, advancing therapies in this area is both scientifically challenging and strategically important. While much of the rare disease development landscape has focused on the U.S. and Europe, Japan is emerging as a leader in creating a supportive environment for rare disease trials and therapeutic innovation.
Government Commitment and Policy Reforms
The Japanese government has made rare and intractable diseases a national priority. Under the Bioeconomy Strategy led by the Cabinet Office, rare diseases are positioned as a key focus area to extend healthy life expectancy and strengthen the healthcare ecosystem.
Several reforms are worth highlighting:
- Regulatory acceleration – Japan’s Ministry of Health, Labour and Welfare (MHLW) and the Pharmaceuticals and Medical Devices Agency (PMDA) are streamlining review processes to speed access for patients. For rare and orphan drugs, this means faster approval pathways and more flexibility in trial design.
- Drug lag reduction – Historically, Japan experienced a delay in the availability of innovative medicines compared to Western markets. Today, regulators are prioritizing alignment with global standards, particularly in rare disease areas where unmet needs are most urgent.
- Bioeconomy integration – By linking healthcare with industrial and academic innovation, Japan is funding translational research and supporting the ecosystem required to bring new therapies to market.
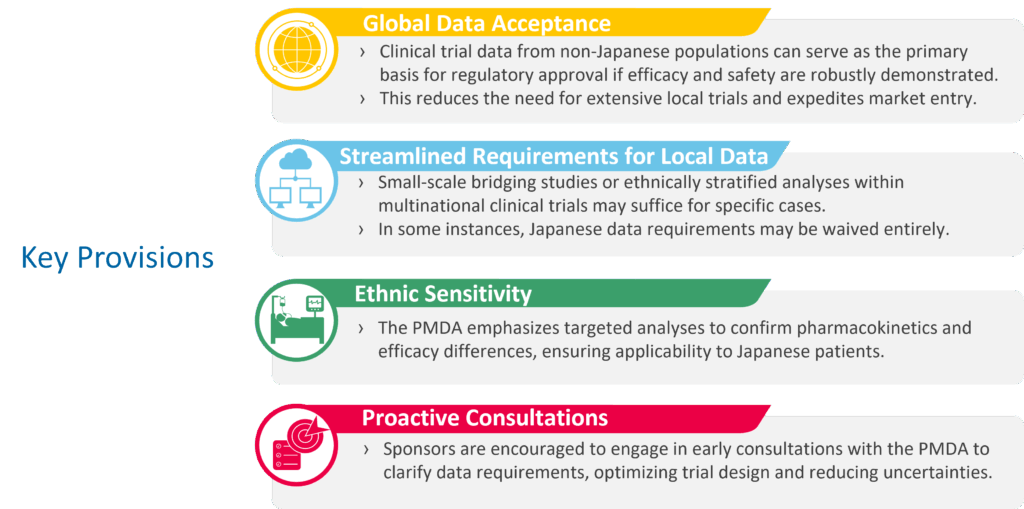
Figure 1: Details on PMDA Updated Regulations
Market Growth and Patient Access
Japan is 2nd for branded drug sales globally and is positioned for significant expansion in biopharmaceuticals and regenerative medicine. The market is projected to grow from USD 9.7 billion in 2020 to USD 21.3 billion in 2030, driven in part by rare and intractable disease investments.
A few factors make Japan especially attractive for rare disease development:
- Universal insurance coverage – Once approved, therapies are reimbursed broadly, enabling access to patients across the country.
- Super-aging population – With over 30% of citizens aged 65 or older, there is a growing demand for therapies addressing rare, age-related, and chronic intractable conditions.
- Patient engagement – Japan has strong networks of hospitals, physicians, and patient advocacy groups, creating an ecosystem supportive of rare disease clinical research.
Clinical Trial Ecosystem
Rare disease studies face challenges such as small patient populations and complex endpoints. Japan has taken steps to support these realities:
- Early regulatory consultation – The PMDA offers frameworks for sponsors to engage early and receive guidance tailored to rare disease trials.
- Multiregional clinical trials (MRCTs) – Japan is increasingly included in MRCTs, allowing global sponsors to incorporate Japanese patients into international programs without duplicating efforts.
- Government-backed investments – Japan continues to fund clinical infrastructure and translational research, strengthening the country’s capacity to host specialized trials. You can learn more about these programs in this brochure.
- Orphan Drug Designation (ODD) – Japan provides ODD incentives such as priority consultation, reduced review timelines, tax credits, and extended re-examination periods to encourage the development of therapies for rare diseases.
- Sakigake Designation – Designed to promote innovative therapies first developed in Japan, Sakigake offers benefits like prioritized consultation, accelerated review, and enhanced support for innovative drugs, medical devices, and regenerative medicines.
Why Partner with CMIC Group
For global companies developing rare disease therapies, Japan presents a rapidly expanding opportunity. But success requires navigating a unique regulatory system, language, and healthcare culture. CMIC Group, Japan’s first and one of the largest Contract Research Organizations (CRO), is the ideal partner to bridge these gaps.
With decades of expertise, CMIC provides regulatory strategy and strong PMDA relationships needed to bring breakthrough therapies to Japanese patients, especially for Orphan Drugs.
For companies in rare disease, now is the time to consider Japan—and CMIC is here to help you make that step.
Contact a representative to schedule a consultation
For more information of Japan Market Entry, download our brochure.
References:
https://www.meti.go.jp/policy/mono_info_service/healthcare
https://www8.cao.go.jp/cstp/bio/index.html
https://www8.cao.go.jp/cstp/bio/bio_sijomap_iyaku.pdf
https://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/orphan_drug.html
https://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/dl/140729-01-02.pdf
