[Blog] Why In-Country Clinical Caretakers Are Essential for Japan Trials
What is an ICCC in Japan?
Conducting clinical trials in Japan as a foreign sponsor requires more than scientific readiness—it demands regulatory alignment with local requirements. One such requirement is the appointment of an In-Country Clinical Caretaker (ICCC), a role that ensures compliance, continuity, and accountability on behalf of overseas sponsors.
Understanding the ICCC Role
An In-Country Clinical Caretaker (ICCC), sometimes referred to as a Clinical Trial In-Country Representative, is an individual or entity residing in Japan who is legally eligible to act on behalf of a sponsor based outside Japan. As outlined by the Japan CRO Association (JCROA), this role is mandated under Japanese Good Clinical Practice (J-GCP) Article 15:
“In order to take the necessary measures to prevent the occurrence or spread of health hazards due to drugs used in the clinical trial, a person who intends to sponsor a clinical trial and resides outside Japan shall appoint a person eligible for sponsoring the clinical trial on behalf of the person who intends to sponsor a clinical trial from among persons residing in Japan (including the head of a Japanese business office of a foreign company) to have him or her (hereinafter referred to as “Clinical Trial In-Country Representative*”) conduct the procedures for sponsoring the clinical trial (J-GCP Article 15). *: Clinical Trial In-Country Representative is referred to as an In-country Clinical Caretaker (ICCC) in this document.”
In practical terms, this means an ICCC manages all sponsor responsibilities locally—including regulatory communications, trial oversight, and risk mitigation—ensuring that Japan’s regulatory standards are met throughout the duration of the trial.
Why the ICCC Matters
Having a qualified and reliable In-Country Clinical Caretaker (ICCC) is critical for:
- Regulatory compliance under J-GCP and all other applicable regulations
- Effective communication with local site staff resulting in efficient trial initiation and site activation
- Effective communication with the PMDA and other authorities
- Timely issue resolution at local trial sites
- Maintaining sponsor oversight despite being based overseas
This is especially important in Japan’s clinical research environment, where cultural nuances and administrative complexity could cause delays or compliance gaps without the right ICCC partner.
ICCC services are a key part of the overall planning to get your drug product to market. Another key service you will need is a Marketing Authorization Holder (MAH), which begins in the filing/approval stage. CMIC offers both these services.
Why Choose CMIC as Your ICCC Partner?
With over 67 ICCC-supported trials in the past five years, CMIC has conducted more ICCC trials than any other domestic CRO in Japan. Our 30+ years of experience and in-depth understanding of the Japanese regulatory landscape make us a trusted choice for global pharmaceutical and biotech sponsors.
At CMIC, we provide more than just ICCC representation—we offer:
- End-to-end clinical trial support, from regulatory consultation to patient enrollment
- Custom-built teams drawing on experts across disciplines
- Proactive project management, issue resolution, and risk mitigation
- Accelerated timelines, backed by local relationships and operational excellence
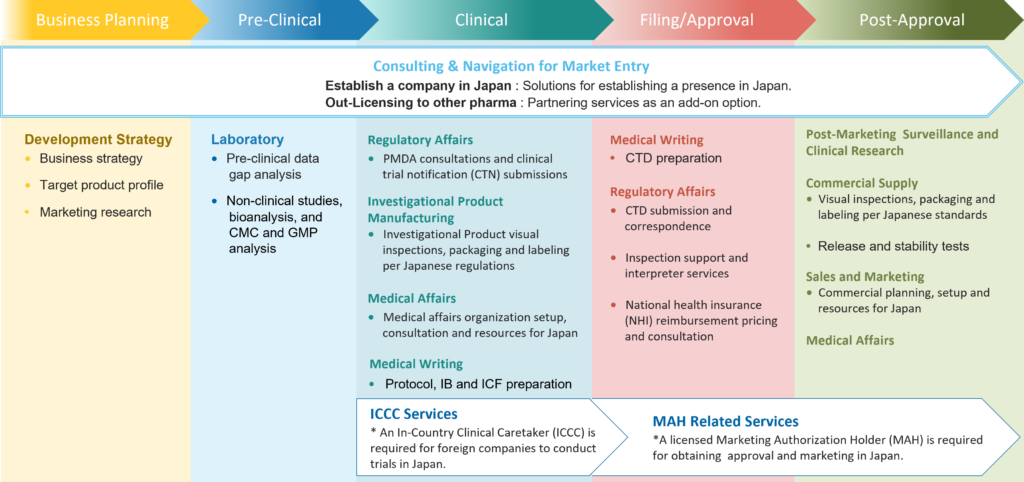
Whether you’re running your first trial in Japan or expanding a global program, CMIC ensures your study is executed with precision, speed, and cultural fluency.
Interested in learning more about ICCC support? Contact us today to explore how CMIC can help bring your clinical trial to life in Japan.
Check out our ICCC dedicated page for further information.
