Is your goal to effectively introduce your product to the market? Do you need a single partner to support development, clinical trial services, commercialization, and more?
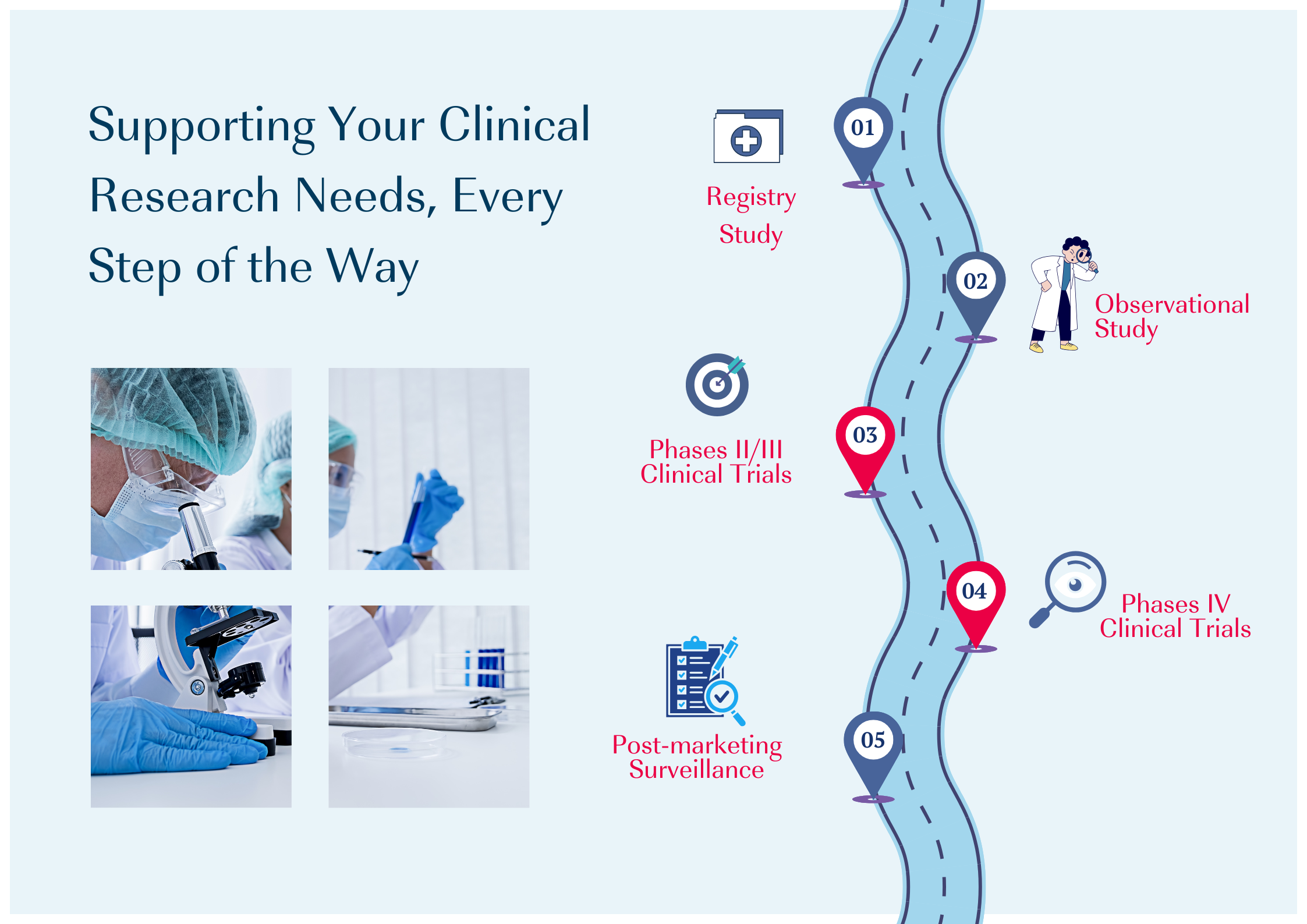
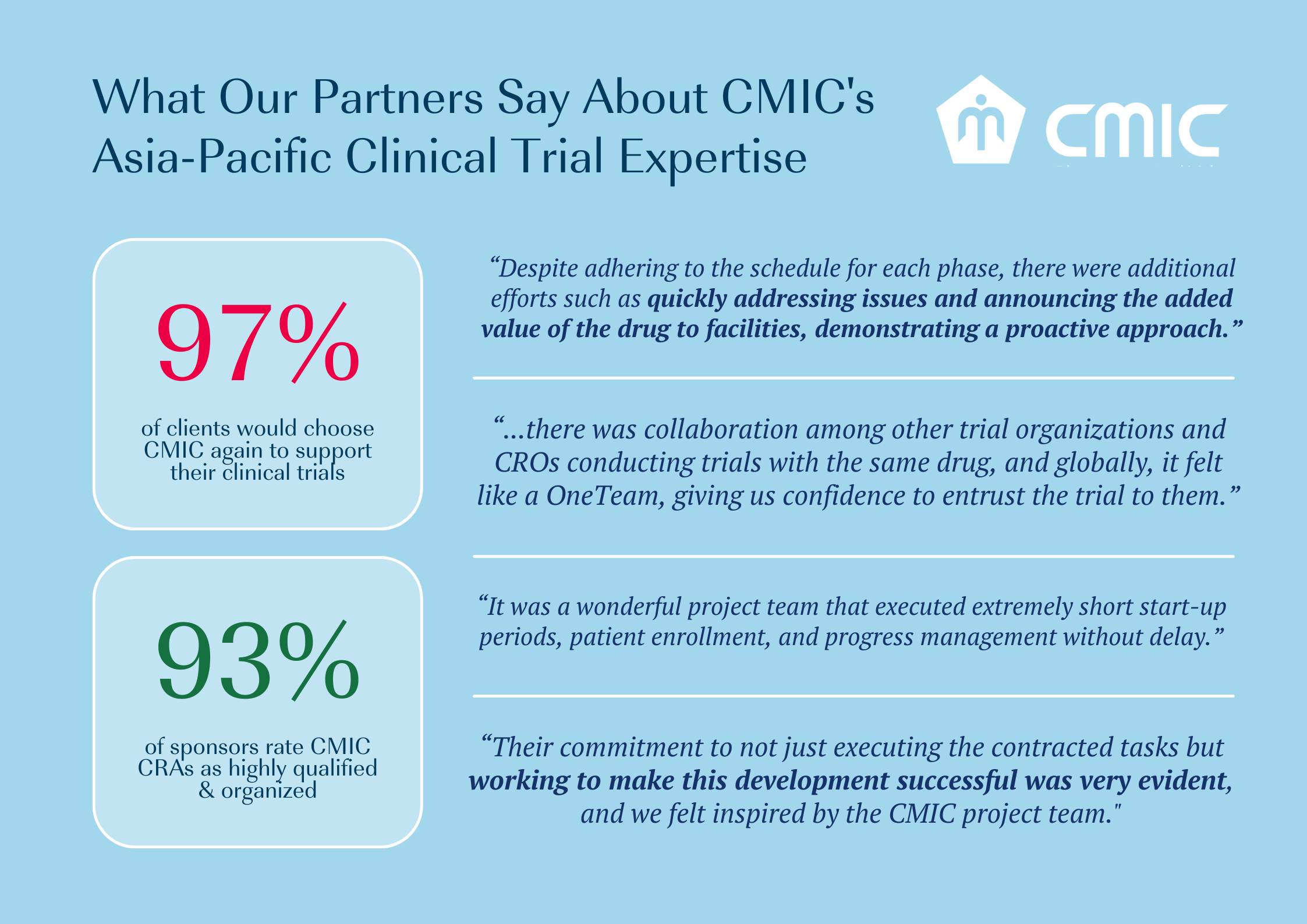
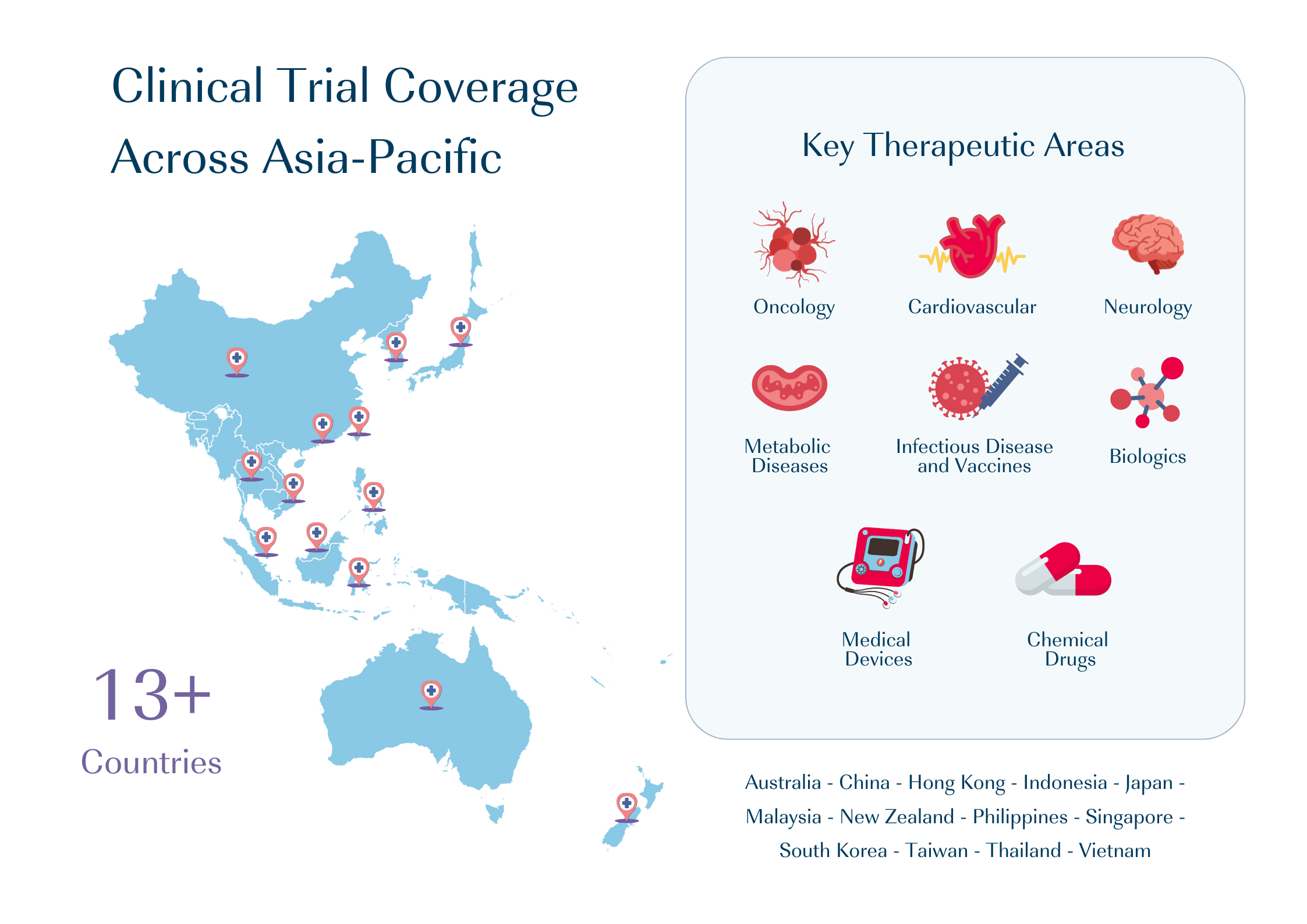
CMIC Group can be your reliable partner. As the largest CRO in Japan, we take pride in supporting clinical trials across more than 13 countries in the Asia South Pacific region. Over the past 5 years, we have supported clinical trials over a range of therapeutic areas.
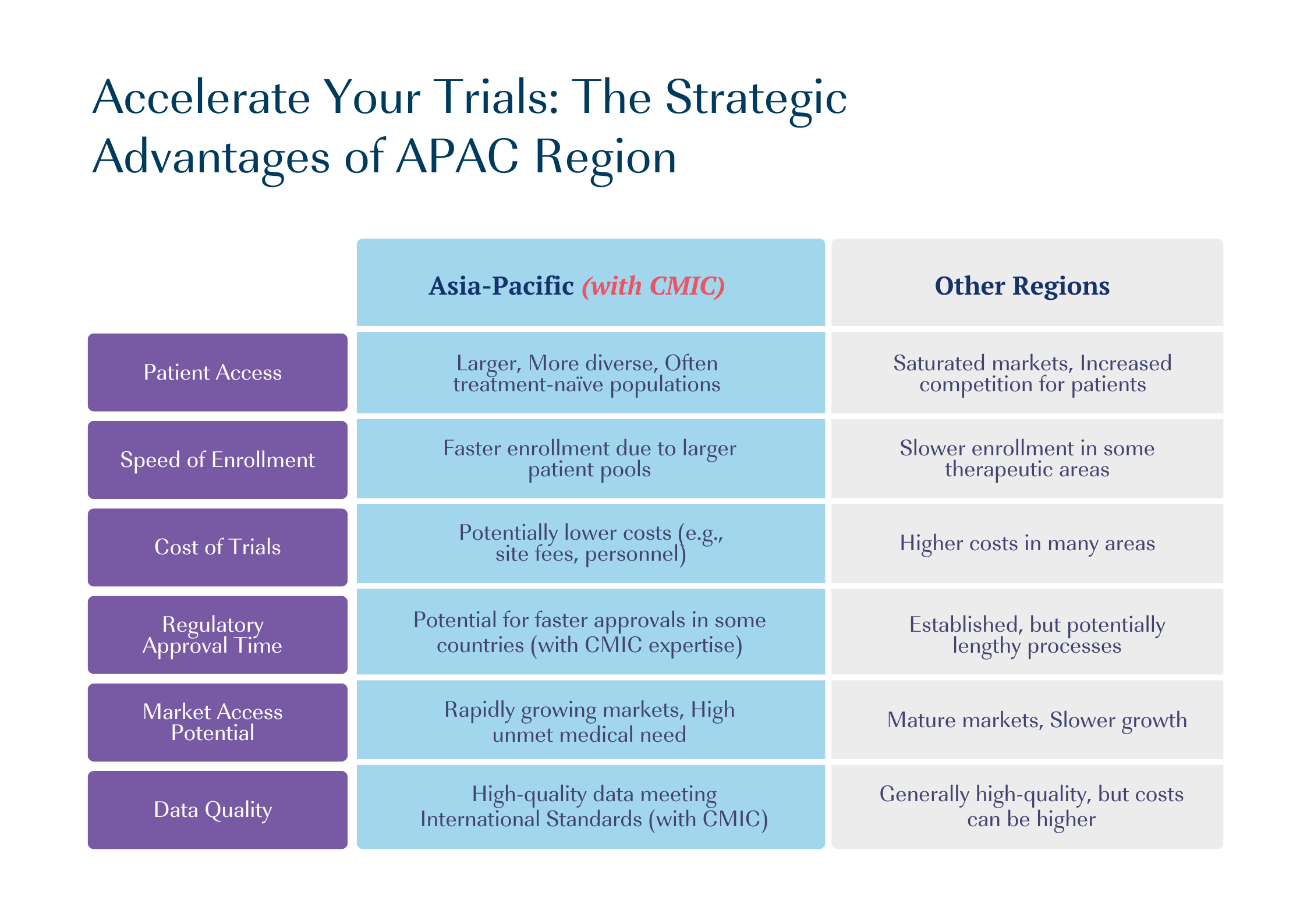
Why consider the Asia Pacific Region for Your Clinical Trials?
The Asia-Pacific region offers several advantages that make it an attractive destination for clinical trials. With its vast population, diverse demographics, and cutting-edge research institutions, an increasing number of pharmaceutical and biotech companies are seeking to conduct clinical trials in Asia.
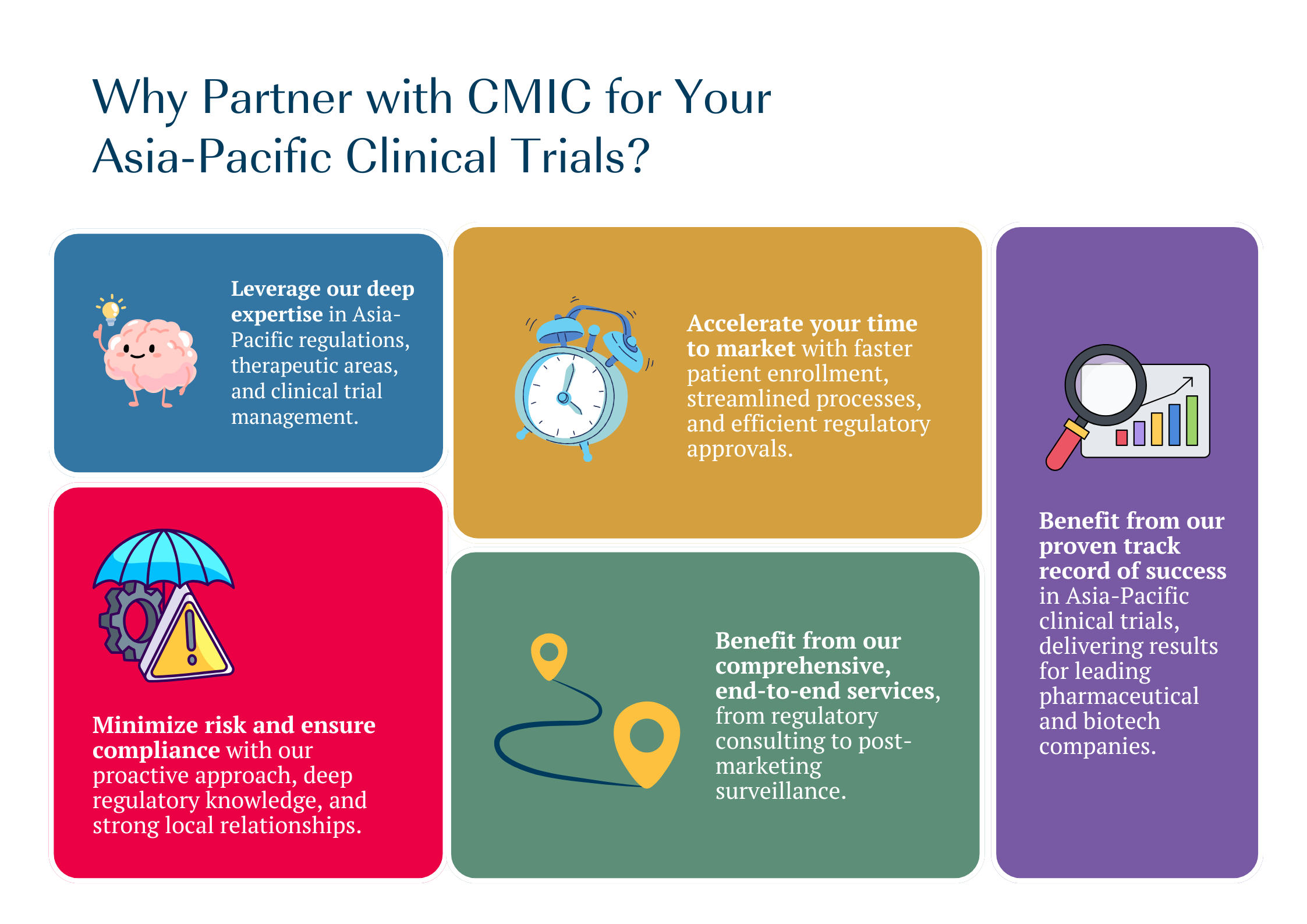
Do you need an expert support for your clinical trials and product commercialization in Japan? Are you looking to have one trusted partner for all stages towards your success?
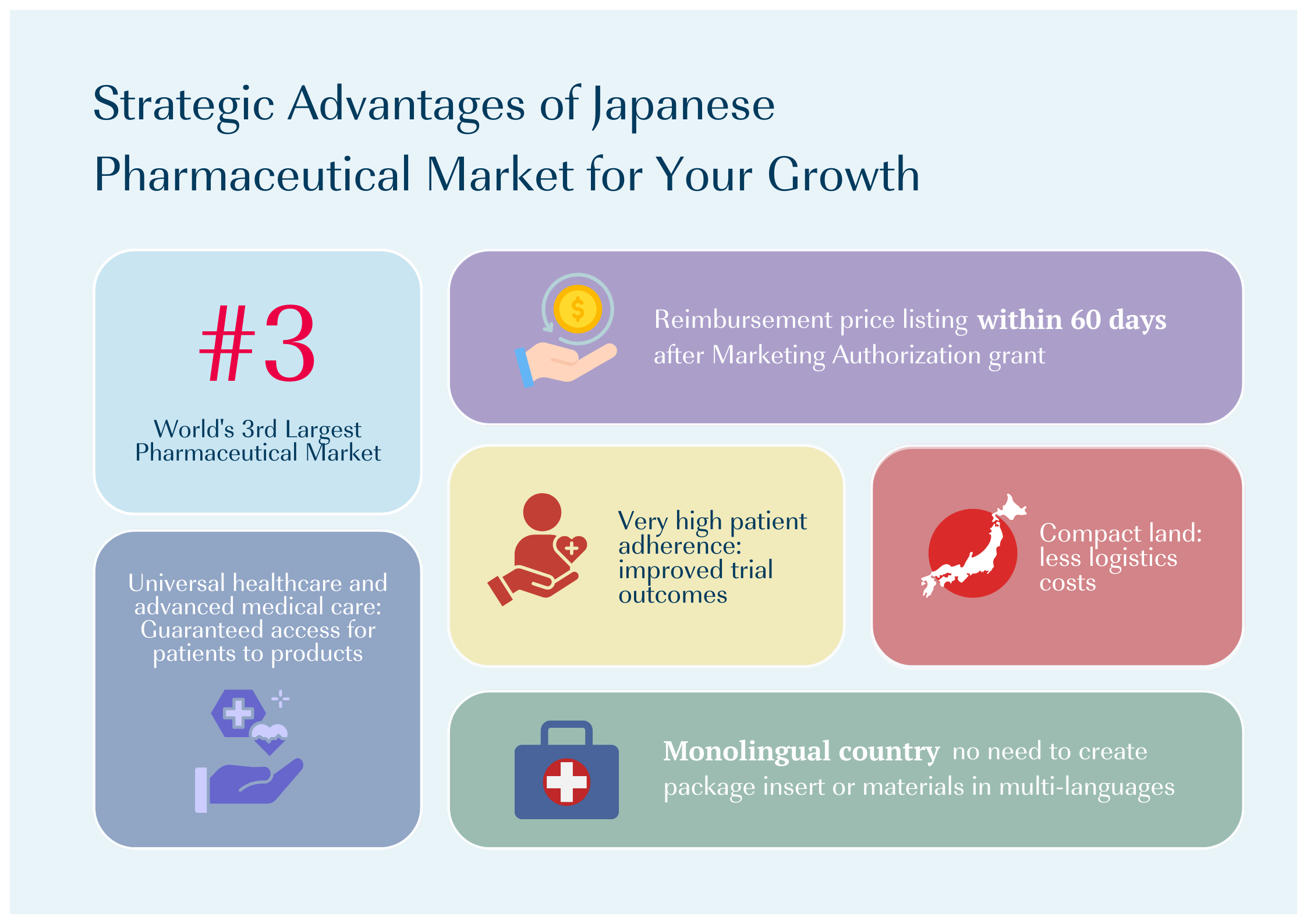
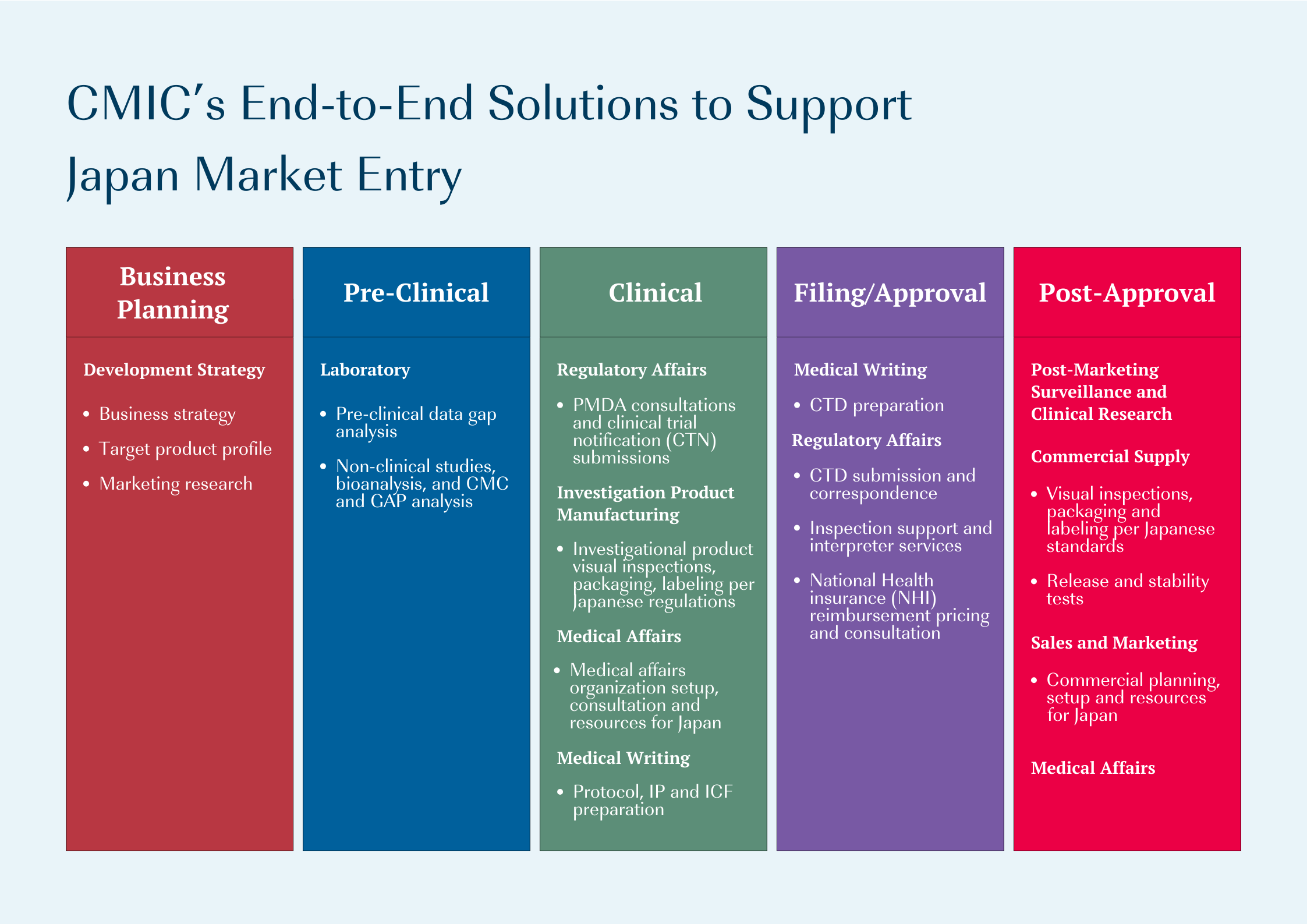
CMIC Group provides full-services support for pharmaceutical companies entering Japan market. Our experienced consultants help you determine the best strategy, navigating Japan’s unique regulations and environment. We offer options for entry at any stage of drug development, whether you’re starting clinical trials, planning regulatory approval, or commercializing an approved product.
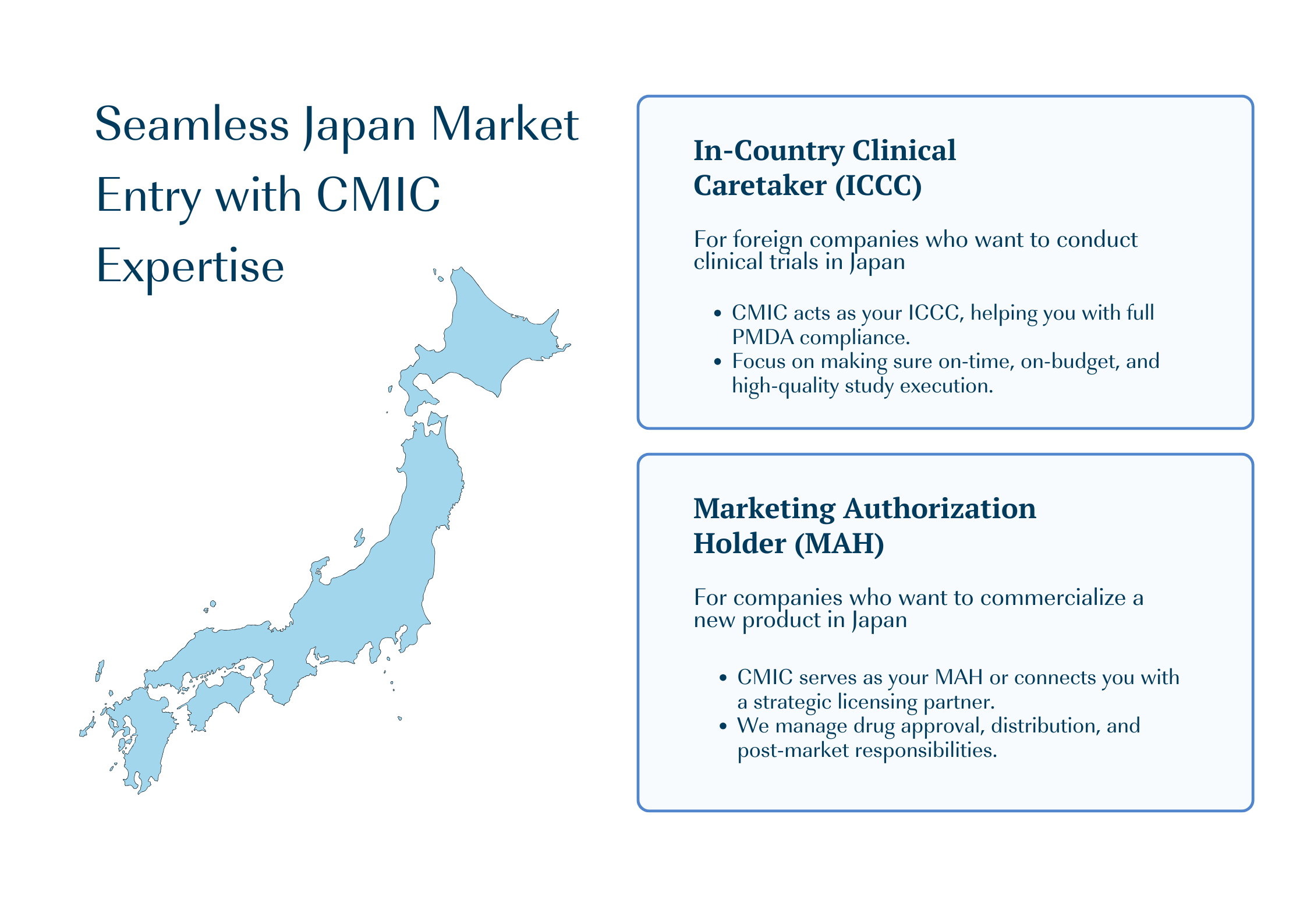
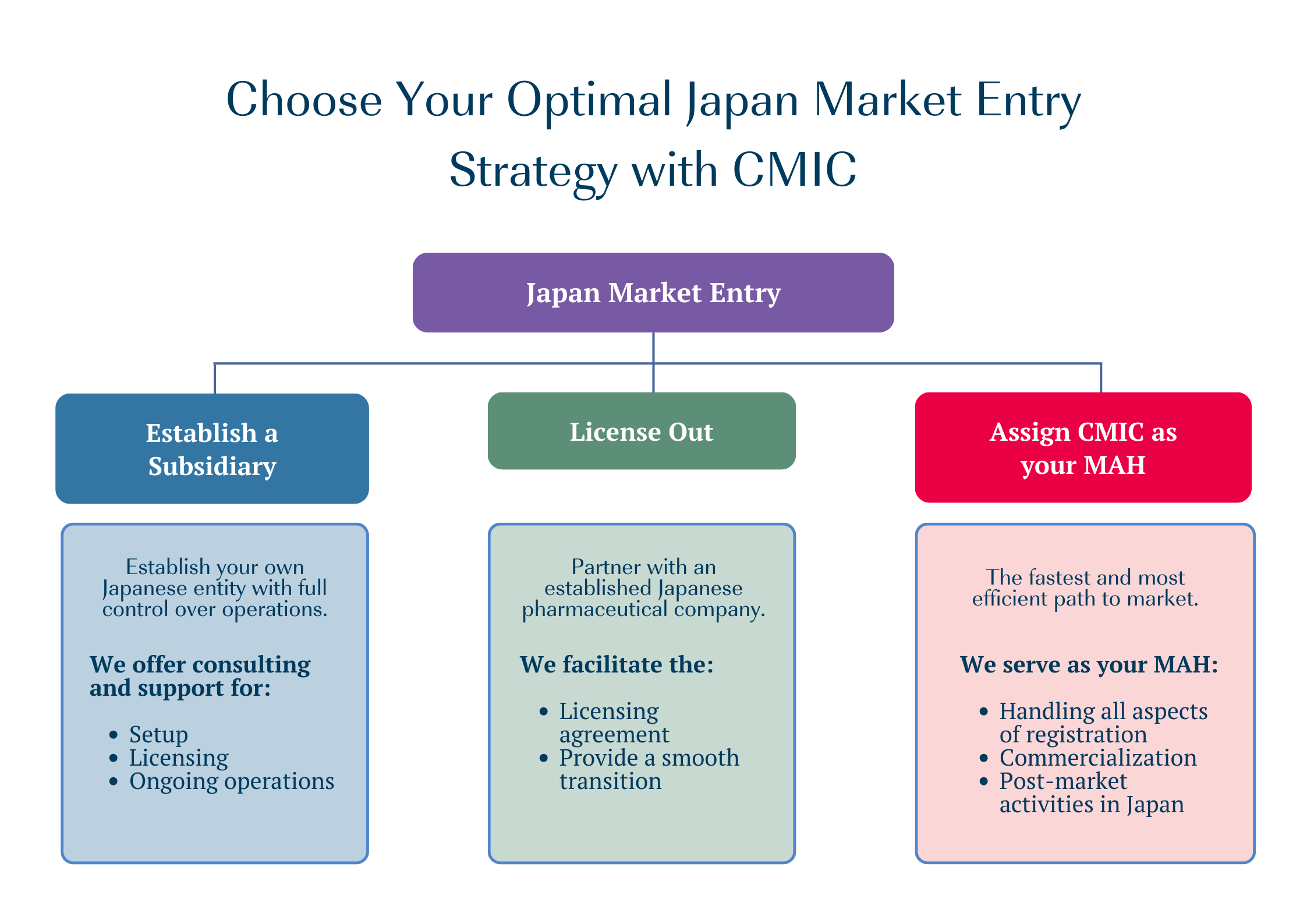
How CMIC Group will Support Your Japan Market Journey?
CMIC can support your Japan market entry no matter which pathway you choose: establishing a subsidiary, licensing out, or assigning CMIC as your MAH.
We are also the only company in Japan offering MAH partnering for orphan drugs through our Orphan Pacific Inc. subsidiary.
Experts from leading clinical sites in Japan, global sponsors and CMIC Group shared insights and real-life examples of patient recruitment, site selection, clinical trial execution and regulatory navigation in Japan.
Don’t miss this opportunity to learn how your company can successfully tap into the Japanese market and accelerate drug development – Watch this free resource today!